Katie Schulz
On the Pulse of PFAS Research
“Just because we live in different jurisdictions doesn’t mean the water cares. It will flow where it flows. And if we’re going to have a sustainable and informed approach to solve some of these problems, it requires that holistic view.”
Meet Katie. She grew up in Milwaukee and knew she wanted a future in science. Katie went to New York City for college. Thinking her trajectory would lead to pre-med, she majored in biology. She ended up in the Big Apple working as a journalist, eventually working full-time for an innovative app-based startup. This was interesting, rewarding work but after the startup folded, Katie found herself drawn again toward a career in science. She investigated her options, including one back home at the UW-Milwaukee School of Freshwater Sciences. Drawn to the environmental health work championed in the labs of Dr. Sandra McLellan and Dr. Rebecca Klaper, Katie interviewed with both leaders and ended up finding a role in Dr. Klaper’s lab. As a master’s student, Katie spent her first year reviewing research (in the parlance of academia—“reviewing the literature” or a “doing a lit review”) on a group of synthetic compounds known collectively as PFAS. You may have heard of them in the news. PFAS are known as “emerging contaminants” because although they’ve been around for some time, they are “emerging” in the sense that scientists, industry, and government are finding that they have unintended effects on life when they get into our environment. Katie is supporting Dr. Klaper and others working to find out more about PFAS and how to avoid the worst health impacts.
After reading well over a hundred research papers on PFAS from other scientists, Katie published her own review paper in an academic journal—an impressive feat as a master’s student—and an important contribution to our shared understanding of these emerging contaminants because even leading researchers may not have the time to read over a hundred papers on a topic. (A “paper” published in an “academic journal” is an often densely written, often lengthy article written in highly technical language which cites other papers the researchers have consulted—reviewing them can be like Alice falling down a rabbit hole.) With the knowledge gained from this review, Katie identified a research project of her own that can add knowledge to the field. She is excited to design and run her own experiment looking at the implications of spreading biosolids on farm fields. (Biosolids are solid byproduct at wastewater treatment plants. Milwaukee’s own Milorganite is an example. Because biosolids are rich in nitrogen they are often used as fertilizer.) Since it is known that PFAS can be found in biosolids, for her second year as a graduate student Katie is exploring how PFAS behave in biosolids. She hopes her work will help inform efforts to regulate the acceptable concentrations to protect our public health.
PFAS are found in almost all human blood and are used in a variety of common and industrial products. Katie explains that they are very useful to society, but their durability is also cause for concern—they were engineered not to break down and they persist at very low concentrations in our environment. They are also associated with health impacts—including thyroid problems and pregnancy complications—at the extremely low concentrations at which they are found. Many contaminants are regulated at the parts-per-million or parts-per-billion level—but the EPA has established 70 parts per trillion as the health advisory level for PFAS in drinking water. The UW-Milwaukee School of Freshwater Sciences is studying how PFAS behave in water and their impacts on aquatic organisms in order to better understand—and address—this emerging public health concern.
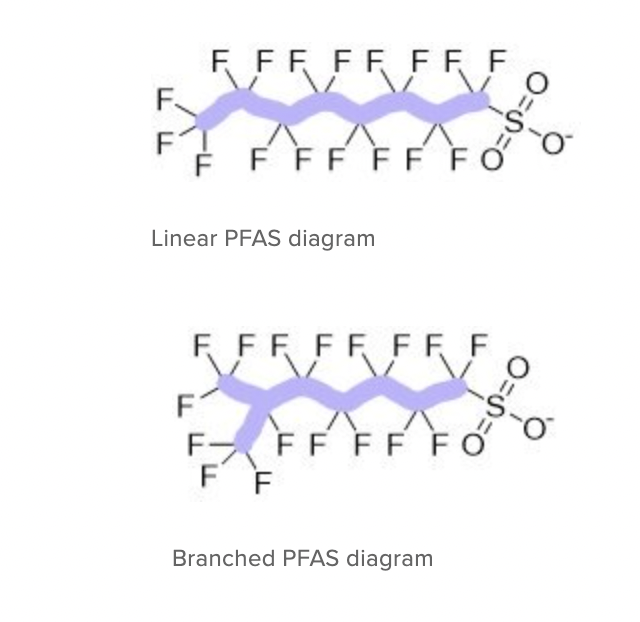
Katie’s literature review delved into the differences between two types of PFAS—those with linear and branched chains. Katie explains that there are a great variety of PFAS, but they are all essentially long carbon chains with fluorine atoms attached along with a functional group that makes them different and useful.
The “linear” isomers have a straight chain. The “branched” isomers are forked. Their shapes have implications for how they behave in the environment and possibly their health impacts. About 30% of PFAS in the environment are “branched.”
Katie found that more linear PFAS tend to be found with soil or sediment, but more branched PFAS tend to be found in surface water and groundwater. This differentiation in how the chemicals react to water has important implications for how and where PFAS may move in the environment. It is knowledge that can help suggest methods to contain or mitigate them.

“For anybody interested in SFS, I think it's a really wonderful program,” Katie says. “What I started out with was looking at all the different faculty members, looking at what their research involves, and starting to think of whose catches your eye, who's studying some facet of water that might be most interesting to you, and then search their name on Google Scholar and look at a couple of their papers. See if what they're doing and have done is interesting and resonates with you, might be something where there's a related avenue worth exploring, and reach out to them, and let them know you've read their research and are interested in learning more, and potentially working with them. The people I reached out to were really receptive to that, despite their really busy schedules. So that's what my advice would be if you're interested in the program.”

PFAS are found in many common and industrial products, including lubricants, fire retardants, and nonstick pans. “Get rid of your scratched nonstick pans,” Katie recommends. “They say not to scratch them or use metal on them, and PFAS is why. If you break the seal on that coating they can start to leach out into your food.”

Katie’s graduate research is considering the implications of PFAS in biosolids, like the sample shown in this flask.
Branched PFAS diagram

